On-Track to Report Top Line Results from Pivotal Phase 3 IMPALA Study at End of Q2 2019
AUSTIN, TX / ACCESSWIRE / March 13, 2018 / Savara Inc. (NASDAQ: SVRA), an orphan lung disease company, today reported financial results for the fourth quarter and full year ending December 31, 2018 and provided a business update.
''With the approaching data read-out from our pivotal IMPALA study, we enter 2019 with confidence,'' said Rob Neville, Chief Executive Officer, Savara. ''We've started key commercial preparation activities to support a potential launch as we believe Molgradex could transform the standard-of-care for a debilitating disease with no approved therapies. We remain focused on becoming the leading orphan lung disease company driving sustainable growth, and delivering significant shareholder value, while advancing the scientific understanding of rare respiratory diseases.''
2018 Developments and Upcoming Highlights
Molgradex for autoimmune pulmonary alveolar proteinosis (aPAP)
- Expect top line results from the IMPALA study at the end of Q2 2019. IMPALA is a global, pivotal Phase 3 clinical study evaluating Molgradex, an inhaled formulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment of aPAP. Positive results would facilitate the submission of a Biologic License Application in the first half of 2020, with an anticipated commercial launch later in 2020 or early 2021.
- Continued strong enrollment in IMPALA-X, an open-label, multicenter extension study to determine the long-term safety and utilization of Molgradex in patients with aPAP. At the end of Q4 2018, 20 out of 21 eligible patients had enrolled in the extension study.
- Announced a partnership with the PAP Foundation to support their efforts to unite, educate and assist the PAP patient community, including work to further expand the PAP patient registry.
Molgradex for nontuberculous mycobacterial (NTM) lung infection
- Announced interim results from OPTIMA, a Phase 2a clinical study evaluating Molgradex for the treatment of NTM lung infection. Early microbiological data showed an encouraging efficacy signal with a favorable safety profile.
- Anticipate top line results in Q1 2020.
- Expect to initiate a Phase 2a study of Molgradex in people living with cystic fibrosis (CF) who have NTM lung infection at the end of Q1 2019 and commence screenings soon after.
AeroVanc
- Continue patient enrollment in AVAIL, a pivotal, global Phase 3 clinical study of AeroVanc, an inhaled vancomycin hydrochloride powder for the treatment of persistent methicillin-resistant Staphylococcus aureus (MRSA) lung infection in CF.
- Expect to complete patient enrollment in Q3 2019 with top line results in Q2 2020. 146 patients are currently enrolled out of a target of 200.
Financials
- Successfully closed a public offering at the end of July 2018 with net proceeds to Savara of approximately $45.8 million.
- Announced Savara's inclusion in the broad-market Russell 3000 Index.
Fourth Quarter Financial Results (Unaudited)
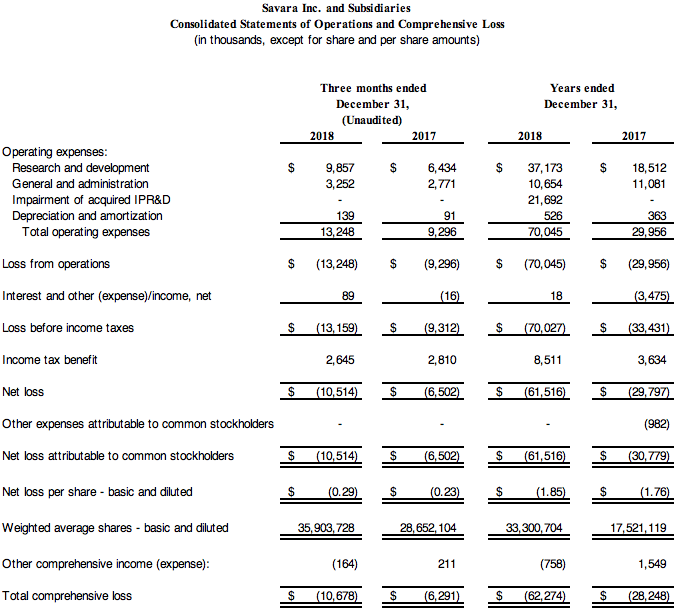
Savara's net loss for the fourth quarter of 2018 was $10.5 million, or $(0.29) per share, compared with a net loss of $6.5 million, or $(0.23) per share, for the fourth quarter of 2017.
Research and development expenses were $9.9 million for the fourth quarter of 2018, compared with $6.4 million for the fourth quarter of 2017.
General and administrative expenses for the fourth quarter of 2018 were $3.3 million, compared with $2.8 million for the fourth quarter of 2017.
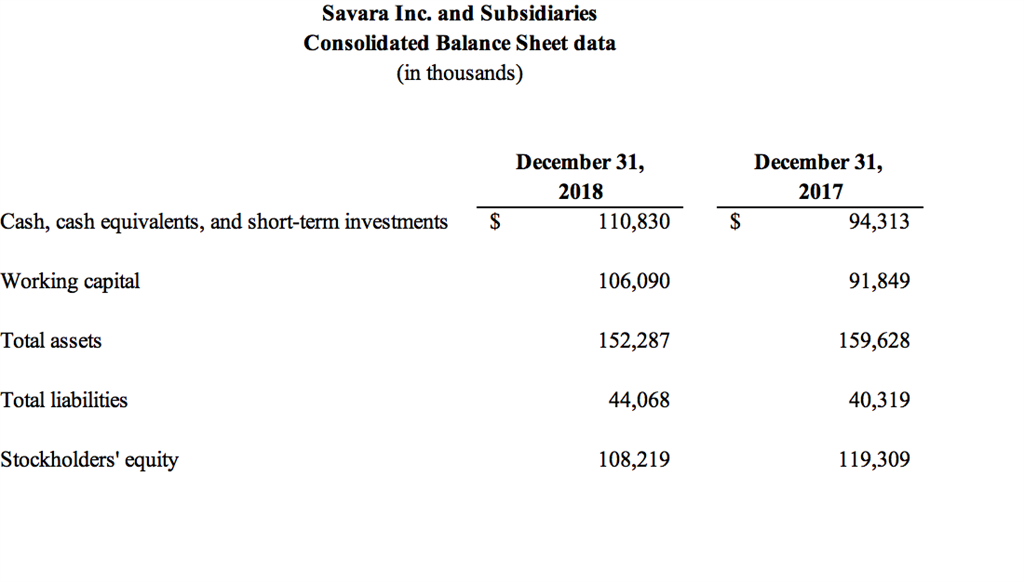
As of December 31, 2018, Savara had cash, cash equivalents and short-term investments of $110.8 million. Savara ended the fourth quarter of 2018 with approximately $24.5 million outstanding in long-term debt used to bolster operations and commercial initiatives and to advance drug candidates.
Fiscal Year 2018 Financial Results
Savara's net loss for the year ended December 31, 2018 was $61.5 million, or $(1.85) per share, compared with a net loss of $29.8 million, or $(1.76) per share for the year ended December 31, 2017.
Research and development expenses increased by $18.7 million, or 101%, to $37.2 million for the year ended December 31, 2018 from $18.5 million for the year ended December 31, 2017. The increase was primarily due to $9.4 million in increased development costs associated with the development of Molgradex, including the expansion of IMPALA in the U.S. and the commencement of OPTIMA, an increase of $8.4 million in AeroVanc study costs related to Phase 3 activities, and $1.0 million in expense for common stock issued to Cardeas Pharma Corporation for the acquisition of their assets.
General and administrative expenses decreased by $0.4 million, or 4.0%, to $10.7 million for the year ended December 31, 2018 from $11.1 million for the year ended December 31, 2017. The decrease was primarily due to $2.0 million of expense in connection with the contingent consideration associated with the acquisition of Molgradex recognized during 2017, offset in 2018 by increased noncash stock-based compensation charges of approximately $1.8 million.
As previously disclosed during Q1 2018, Savara recognized a $21.7 million impairment charge to the carrying value of In-Process Research and Development related to the Aironite drug candidate assumed in the April 2017 merger with Mast Therapeutics, Inc. Savara reduced the associated deferred tax liability by $4.6 million and recorded an income tax benefit.
Conference Call and Webcast
Savara will hold a conference call today beginning at 5:30 PM Eastern Time/2:30 PM Pacific Time to provide a business update. Shareholders and other interested parties may access the conference call by dialing (855) 239-3120 from the U.S., (855) 669-9657 from Canada, and (412) 542-4127 from elsewhere outside the U.S. and request the ''Savara Inc.'' call. A live webcast of the conference call will be available online in the Investors section of Savara's website at https://www.savarapharma.com/investors/events/. Approximately one hour after the call, a replay of the webcast will be available on Savara's website for 30 days, and a telephone replay will be available through March 20, 2019 by dialing (877) 344-7529 from the U.S., (855) 669-9658 from Canada and (412) 317-0088 from elsewhere outside the U.S. and entering the replay access code 10128884.
About Savara
Savara is an orphan lung disease company. Savara's pipeline comprises Molgradex, an inhaled granulocyte-macrophage colony-stimulating factor (GM-CSF) in Phase 3 development for autoimmune pulmonary alveolar proteinosis (aPAP), in Phase 2a development for nontuberculous mycobacterial (NTM) lung infection, and in preparation for Phase 2a development in cystic fibrosis (CF) affected individuals with chronic NTM lung infection; and AeroVanc, a Phase 3-stage inhaled vancomycin for treatment of persistent methicillin-resistant Staphylococcus aureus (MRSA) lung infection in CF. Savara's strategy involves expanding its pipeline of potentially best-in-class products through indication expansion, strategic development partnerships and product acquisitions, with the goal of becoming a leading company in its field. The most recent acquisition is aerosolized amikacin/fosfomycin, a Phase 2-ready, proprietary combination antibiotic, which has demonstrated potent and broad-spectrum antibacterial activity against highly drug resistant pathogens. Savara's management team has significant experience in orphan drug development and pulmonary medicine, identifying unmet needs, developing and acquiring new product candidates, and effectively advancing them to approvals and commercialization. More information can be found at www.savarapharma.com. (Twitter: @SavaraPharma, LinkedIn: www.linkedin.com/company/savara-pharmaceuticals/)
Forward Looking Statements
Savara cautions you that statements in this press release that are not a description of historical fact are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words referencing future events or circumstances such as ''expect,'' ''intend,'' ''plan,'' ''anticipate,'' ''believe,'' and ''will,'' among others. Such statements include, but are not limited to, our belief that Molgradex could transform the standard-of-care for a debilitating disease with no approved therapies, statements regarding our focus on becoming the leading orphan lung disease company driving sustainable growth, and delivering significant shareholder value, while advancing the scientific understanding of rare respiratory diseases, statements regarding the timing of top line results from our IMPALA, OPTIMA and AVAIL studies, that positive results from IMPALA would facilitate the submission of a Biologics License Application in the first half of 2020, with an anticipated commercial launch in the U.S. in 2020 or early 2021, statements regarding the enrollment of our IMPALA-X study, statements regarding our support of the efforts of the PAP Foundation to unite, educate and assist the PAP patient community, including work to further expand the PAP patient registry, statements regarding the enrollment of our AVAIL study, including the timing of completion of enrollment, that we expect to initiate a Phase 2a study of Molgradex in people living with cystic fibrosis who have NTM lung infection at the end of Q1 2019 and commence screenings soon after, and Savara's strategy. Savara may not actually achieve any of the matters referred to in such forward-looking statements, and you should not place undue reliance on these forward-looking statements. These forward-looking statements are based upon Savara's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with the outcome of our ongoing and planned clinical trials for our product candidates, the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources for Savara's operations and to conduct or continue planned clinical development programs, the ability to obtain the necessary patient enrollment for our product candidates in a timely manner, the ability to successfully identify product acquisition candidates, the ability to successfully develop our product candidates, the risks associated with the process of developing, obtaining regulatory approval for and commercializing drug candidates such as Molgradex, AeroVanc and amikacin/fosfomycin that are safe and effective for use as human therapeutics and the timing and ability of Savara to raise additional equity capital as needed to fund continued operations. All forward-looking statements are expressly qualified in their entirety by these cautionary statements. For a detailed description of our risks and uncertainties, you are encouraged to review our documents filed with the SEC including our recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they were made. Savara undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as may be required by law.
Financial Information to Follow
Contacts:
Savara Inc. IR/PR:
Anne Erickson ([email protected])
(512) 851-1366
For IR: Solebury Trout
Gitanjali Jain Ogawa ([email protected])
(646) 378-2949
SOURCE: Savara Inc.