3RD PARTY TESTING AND ANALYSIS OF COMPANY'S GENULTIMATE TBG REVEAL NEVER BEFORE SEEN PRECISION AND ACCURACY AT LEVELS 60% BETTER THAN FDA AND ISO PUBLISHED STANDARDS
LOS ANGELES, CA / ACCESSWIRE / September 19, 2019 / Decision Diagnostics Corp. (OTC PINK:DECN) is a 17-year old, diabetes-focused bio-technology development firm, manufacturer, quality plan administrator, FDA registered medical device customer support organization, and exclusive worldwide sales and regulatory process agent for the GenUltimate! ("Sunshine") diabetes test strip, the GenSure! ("Feather") diabetes test strip for International markets, and its GenChoice! ("Ladybug") test strip now in FDA 510(k) prosecution. The company also markets its PetSure! test strip for the diabetic testing of dogs and cats, a diagnostic specifically designed to run on the market leading Zoetis Alpha Trak meter system and the GenUltimate! 4Pets Test strip and Avantage! meter a proprietary testing product for dogs, cats and horses, and the panacea GenUltimate! TBG ("Dragonfly") diabetes testing system, now awaiting a clinical trial slot in Korea, expected in late October 2019.
DECN announces today that it has completed final development and third party testing of its panacea GenUltimate TBG technology, now added to the company's Quality System, including the Design History, PDR and Design Master Record files. Subsequently, DECN has retained counsel to prosecute applications for two patents as the company moves quickly to secure its diagnostic technology. Work on the patent applications began earlier in September.

Keith Berman, CEO of DECN commented, "The latest and final revision of GenUltimate TBG has been provided to the company, and we have enthusiastically accepted TBG into our stable of products. Work will begin immediately to file two patents, the first a patent on the TBG version of the GenUltimate test strip, and a second patent on the TBG system which includes our new Precise! meter."
Mr. Berman continues, "The company made what it considered to be a minor change to the TBG chemistry, but this minor change produced breathtaking results, a true "take notice" moment. Our proposed licensing partner has certainly taken notice. With all of the action in the industry focused on Continuous Glucose Monitoring devices, a market we are also investigating at present, we decided to focus our near term attention on our own technology, change our business direction without changing our business model, and as a result we have taken our technology to its precise conclusion. Frankly, in our opinions, GenUltimate TBG is the best commercial glucose test strip ever fulfilled.
The first implementations for the TGB chemistries will be for the company's GenUltimate! and GenUltimate Zephyr! products. Both of these products run on the venerable Lifescan OneTouch Ultra family of glucometers. They say, a picture is worth 1000 words. The pictures below best describe our TGB implementation."
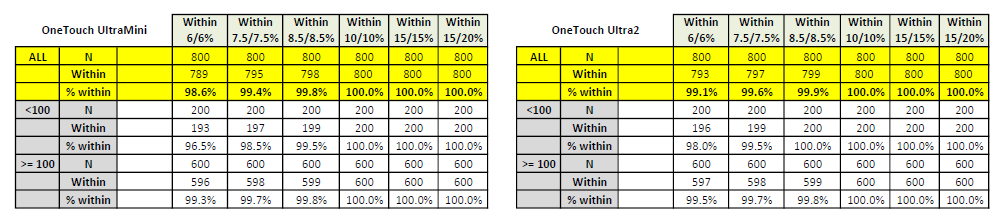
Mr. Berman concluded, "The data displayed above is just a small summary portion of the volumes of testing data available for GenUltimate TGB. We have posted the full summary of the study on one of our Web Sites. Please follow the link below. To view all of the data we have posted, you will have to send an email to [email protected]. Ask for the password in your email.
http://www.decisiondiagnostics.co/docs/new/2019.09.16%20GenUltimate%20TBG_GDH.pdf
ABOUT DECISION DIAGNOSTICS CORP
Decision Diagnostics Corp. is the leading manufacturer and worldwide distributor of diabetic test strips engineered to operate on legacy glucose meters. DECN's products are designed to operate efficiently and less expensively on certain glucose meters already in use by almost 7.5 million diabetics worldwide. With new inspired technology diabetic test strips already in the final stages of development, DECN products compete on a worldwide scale with legacy manufacturers currently selling to 71+ percent of a $15+ billion at-home testing market. The company's GenUltimate TBG product is not yet available for sale in the United States or Puerto Rico.
Forward-Looking Statements:
This release contains the company's forward-looking statements which are based on management's current expectations and assumptions as of September 18, 2019, regarding the company's business and performance, its prospects, current factors, the economy, and other future conditions and forecasts of future events, circumstances, and results.
CONTACT INFORMATION:
Decision Diagnostics Corp.
Keith Berman (805) 446-2973
[email protected]
www.genultimate.com
www.genultimatetbg.com
www.petsureteststrips.com
www.pharmatechdirect.com
SOURCE: Decision Diagnostics Corp.




