Company will offer choice of Covid-19 Kits to be sold Internationally, and then in the U.S. after receipt of FDA Authorization, as Saliva Based Kit is not anticipated to require changes to GenViro! Swift Test Strip or Meter except for Software Addition
LOS ANGELES, CA / ACCESSWIRE / July 10, 2020 / Decision Diagnostics Corp. (OTC PINK:DECN) through its subsidiary Pharma Tech Solutions, Inc., today announced it plans to provide a new single-use saliva testing kit option to its professional and individual use finger stick GenViro! Covid 19 Swift Kits designed to identify Covid-19 viral load. Test reporting for the GenViro! finger stick kits are currently producing results at :10.5 seconds, and initial testing completed on the saliva version of the kit should yield even faster results since the saliva testing will not require any sample correction. The GenViro! Saliva Covid-19 Swift Kit is non-invasive. In consultation with the FDA, the company will either work to supplement its two current EUA applications, device (serial number) PEUA200232, GenViro! Covid-19 Swift Kit for professional use in commercial and group settings and device (serial number) PEUA200947, GenViro! Covid-19 Screening Kit for individual at-home use, or file two new applications.
Preliminary testing, some illustrated below, was run using saliva from human donors and indicated that the saliva exhibits a comparable, and in fact favorable, impedance curve profile when compared to whole blood. In addition, saliva does not contain hematocrit (an abundance of or non-abundance of red blood cells) which on our whole blood based product required adapting our patent-pending TBG technology to the GenViro! test strip. Smooth and linear impedance curves are critical because our methods are designed to measure viral load at specific impedance frequencies (see Graph 1: Phase) and then report this load factor (see Graph 2: Magnitude). Data was compiled at our South Korean contract manufacturing and product development site at DECN's request.
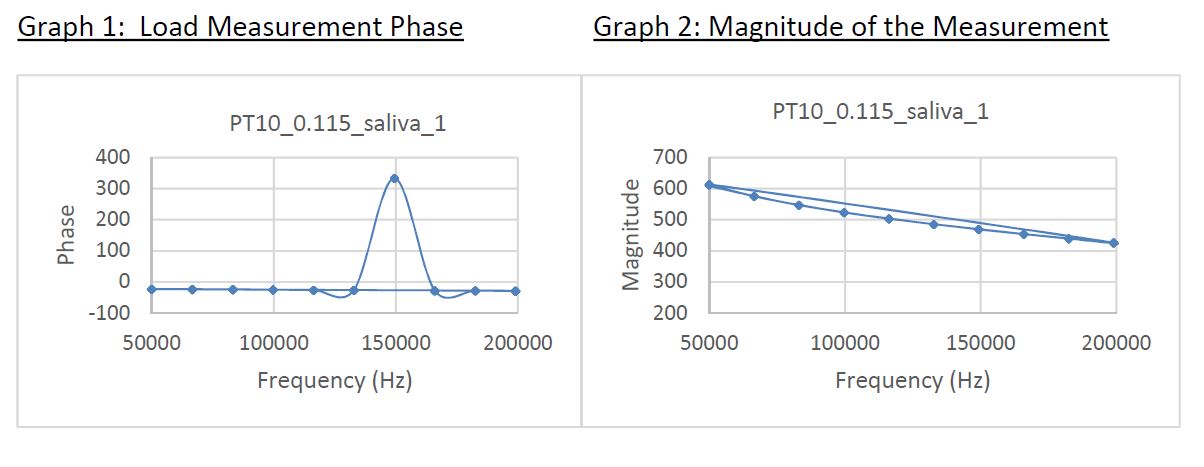
Initial feasibility testing of the saliva modality proved extremely positive.
The company's two EUA applications are currently under FDA review while final interviews to select a clinical trial partner, needed to meet FDA new data foundations are in motion. The first task of the clinical trials organization is to produce a detailed clinical trials testing protocol. This is a short term task. There will be different protocols for the whole blood and saliva clinicals. The company intends to test GenViro! two ways; first using contrived blood or virus transfer media to complete the process for anticipated international sales, and then testing with live patients using a modification to the FDA's 30/75 formula. DECN intends to test 40 or more known infected patients to minimize any effects from statistical outliers.
Self-collection testing methodologies producing immediate results will be a critical factor in identifying who is currently infected with the virus, and who therefore needs to be quarantined. Experts agree that being able to have an adequate number of people around the globe and in the U.S. self-test, whether the test is administered at some centralized location or in private at home, and to do so on a consistent and continual basis, is a key to controlling and ultimately beating the Covid-19 pandemic. Since both the original finger stick and new saliva methods use the GenViro! Swift test strips and reader device, the addition of the saliva test option should have no impact on the current test strip design or final development and testing schedule. Both methods are expected to provide the accuracy, immediacy and affordability needed to help control the spread of the virus.
"Offering a second, independent, rapid, safe and reliable method of professional point-of-care and self-testing home kit for Covid-19 is potentially transformative in so far as identifying those who are infected and being able to take appropriate measures to address their needs, protect others and prevent spreading," said DECN CEO Keith Berman. "While the finger-stick method has been successfully used in diabetic testing for decades, and represents our core competency, we felt that providing a non-invasive option for Covid-19 testing might be more appealing to both the patient and the professional. We also believe it might be a more desirable option for use by businesses during re-openings, at large venues, at sporting events, at places of worship, and in fact in almost any circumstance one could think of where testing providing immediate results would be prudent, if not mandatory. We strongly believe in the two testing modalities and see a huge benefit for persons across the US and the world."
While the FDA has already authorized several Covid-19 saliva tests that are on the market, none of these products offer true point of care results like DECN's products will. The primary criticism of those current testing methods is that they take too long to provide test results, sometimes requiring those tested to wait days for a result. By the time the results are provided, the patient, if positive but undiagnosed, could have already spread the virus to many others. Both DECN Genviro! Swift Kits will include everything that is required for professionals and individuals to test, including detailed instructions in multiple languages and a video.
ABOUT DECISION DIAGNOSTICS CORP
Decision Diagnostics Corp. has been the leading manufacturer and worldwide distributor of diabetic test strips engineered to operate on legacy glucose meters for 18 years. DECN's products are designed to operate efficiently and less expensively on certain glucose meters already in use by almost 7.5 million diabetics worldwide. The company's GenViro!™ products are designed to test for Covid-19, and applications for Emergency (EUA) Waivers have been submitted to the U.S. FDA. The finger-stick test kit is currently being readied for international sales and an agreement for distribution has been signed for sales to commence in multiple countries including India, Malaysia, Singapore, Nepal, Bangladesh, Sri Lanka, Indonesia, Thailand, Vietnam and Australia. Registration in those countries where such documentation is required is the responsibility of the distributor.
Forward-Looking Statements:
This release contains the company's forward-looking statements which are based on management's current expectations and assumptions as of July 9, 2020, regarding the company's business and performance, its prospects, current factors, the economy, and other future conditions and forecasts of future events, circumstances, and results.
CONTACT INFORMATION:
Decision Diagnostics Corp.
Keith Berman
(805) 446-2973
[email protected]
www.genultimate.com
www.genultimatetbg.com
www.pharmatechdirect.com
SOURCE: Decision Diagnostics Corp.




