TORONTO, ON / ACCESSWIRE / August 19, 2020 / Theralase® Technologies Inc. ("Theralase" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company focused on the research and development of light activated Photo Dynamic Compounds ("PDC") and their associated drug formulations to safely and effectively destroy various cancers, announced today that it has executed a Sponsored Research Agreement ("SRA") with the University of Manitoba ("UM") Medical Microbiology department to commence development of a coronavirus vaccine and therapy utilizing Theralase's patented and proprietary PDCs. According to the SRA, UM will conduct experiments in conjunction with Theralase for the research and development of a coronavirus vaccine and therapeutic to be further evaluated in animal then human clinical testing in 2021.
Dr. Kevin Coombs, PhD, Professor, Department of Medical Microbiology, will lead the research for the University of Manitoba. One of Dr. Coombs' interests is the investigation of how COVID-19 impacts genes and proteins in lung cells. In addition to Theralase's anti-COVID research, Dr. Coombs will lead a multi-institutional consortium using a powerful research tool, called SomaScan, and next-generation sequencing, to rapidly determine how COVID-19 (SARS-CoV-2 coronavirus) - and a variety of other coronaviruses - affect large numbers of genes and proteins in different human cells, which are the normal target of the COVID-19 virus.
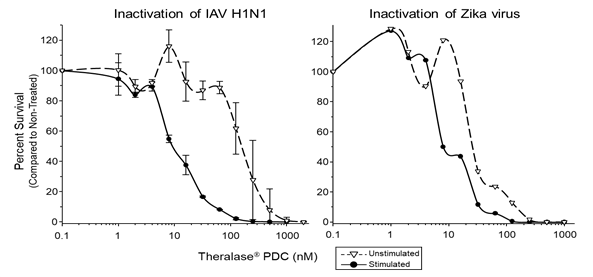
Dr Coombs stated, "I have had the opportunity to independently evaluate Theralase's PDCs, on an in-vitro pre-clinical basis, as a potential new anti-viral drug; specifically targeting COVID-19. I have completed a preliminary analysis of how they affect two enveloped viruses very similar in make-up to COVID-19; specifically H1N1 Influenza and Zika virus, and am impressed with the high efficacy kill rates, both with and without stimulation."
Dr. Coombs went on to say, "From this initial data, Theralase's PDCs have a very high anti-viral activity at a very low concentration, in the nanomolar range, even when not stimulated. It is also noteworthy that this effect was observed at concentrations well below toxicity levels observed in mammalian cells or mammalian patients; therefore, this approach would provide a very high efficacy to safety ratio. Thus, the PDCs effective concentration is comparable to, or better than, many other anti-virals that my lab has tested and this virus killing capacity is further improved when the PDC is light activated, alone or in various formulations."
Dr Coombs also stated "Based upon the above results, I believe further studies are strongly warranted. Theralases's PDCs show great promise as a potential therapeutic. Given the similar physicochemical properties of all enveloped viruses, including the new coronavirus SARS-CoV-2, I believe Theralase's PDCs represent an important new anti-viral technology platform and I am very interested in further exploring how these PDCs could be used against COVID-19." Dr. Coombs continued, "We are planning to use a Direct Electron Detector ("DED") enhancement to a powerful TALOS200 electron microscope, which allows multichannel, high-resolution imaging and precise compositional analysis to enable dynamic microscopy applications at sub-nanometer resolution. I will use this technology to look at structures of coronaviruses after they have been treated with Theralase's formulations for cellular destruction. I look forward to working with Dr. Arkady Mandel and the Theralase team in the development of this exciting technology."
Dr. Arkady Mandel, Chief Scientific Officer, Theralase Technologies Inc. stated. "As the deadly new coronavirus continues to spread globally, Theralase has originated and fostered development of an advanced vaccine and therapy based on standardized anti-viral approaches by optimising its PDCs and anti-cancer technology platforms to prevent and fight enveloped viruses, including coronaviruses, such as COVID-19. We are pleased to announce very promising early results of our research that has been conducted by Dr. Coombs and his team at UM. This is a global pandemic, which requires research partnerships and the combined strength of multidisciplinary teams. By joining forces with UM, Theralase believes it can develop an elegant solution that will help mitigate this threat quickly and efficiently. Data in four preclinical models in both infectious disease and oncology strongly suggests that the Theralase technology platform has flexibility and bandwidth to be clinically relevant in destruction of numerous cancers and pathogens, including enveloped viruses. Now, with a new pandemic raging, Theralase is working on the development of effective mitigation strategies for the novel coronavirus that encompasses prevention of the disease and effective treatment of patients who contract COVID-19. Working with our partners at UM, the Company looks forward to introducing this game changing technology to the world in due course."
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers.
Additional information is available at www.theralase.com and www.sedar.com
Forward Looking Statement:
This news release contains "forward-looking statements" which reflect the current expectations of Company's management for future growth, results of operations, performance, business prospects and opportunities. Such statements include, but are not limited to, statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as "may", "would", "could", "should", "will", "anticipate", "believe", "plan", "expect", "intend", "estimate", "potential for" and similar expressions have been used to identify these forward-looking statements. These statements reflect management's beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions; including, with respect to the ability of the Company to: adequately fund, secure the requisite regulatory approvals to commence and successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its commercialization plans. Many factors could cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Company with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward-looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully, and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Company disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273)
416-699-LASE (5273)
www.theralase.com
Kristina Hachey
Chief Financial Officer
[email protected]
416-699-LASE (5273) x 224
SOURCE: Theralase® Technologies Inc.




