VELDONA® formulation showed significant stabilization of physical condition and complete recovery from lung inflammation throughout the course of treatment period.
By upregulation of immunomodulatory, Ainos' VELDONA® formulation demonstrates efficacy against new variant virus based on clinical symptoms and lung inflammation.
SAN DIEGO, CA / ACCESSWIRE / September 30, 2022 / Ainos, Inc. (NASDAQ:AIMD, AIMDW) ("Ainos", or the "Company"), a diversified medtech company focused on the development of novel point-of-care testing, low-dose interferon therapeutics, and synthetic RNA-driven preventative medicine, today announced the results from its antiviral efficacy study in hamsters against the Omicron variant of SARS-CoV-2 (the "Study"). The Company's results showed that its low-dose oral interferon alpha ("IFN-α") formulation, VELDONA®, had a therapeutic effect on lungs infected with the SARS-CoV-2 (the "Omicron variant") virus by regulating the immune response, thereby expediting recovery of infected animals. The Company has submitted the application documents for U.S. FDA Phase 2 clinical trials for evaluating the efficacy of VELDONA® in patients with mild COVID-19. Further information regarding the Study can be found on Ainos' website (link).
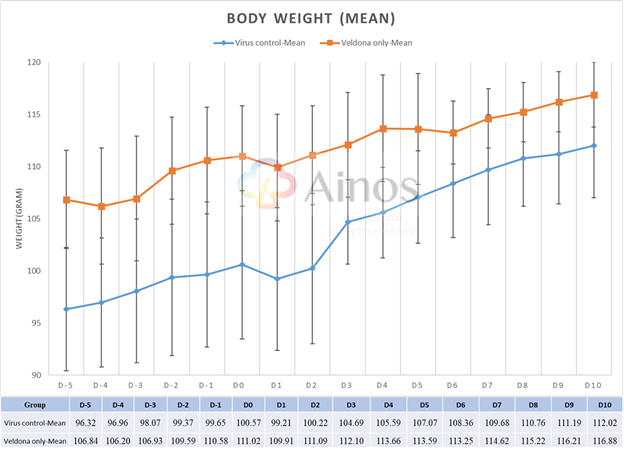
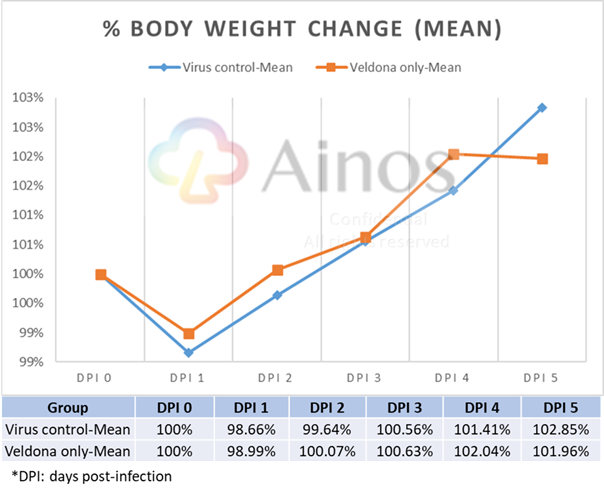
The Study evaluated the effectiveness of VELDONA® over a sixteen-day course (five-day pre-treatment, one-day during viral infection and ten-day treatment after infection) of Omicron-variant-infected hamsters. Compared with hamsters in the placebo group receiving solution without VELDONA® (the "Placebo Group"), the hamsters in the group receiving solution with VELDONA® (the "VELDONA® Group") demonstrated resistance to body weight loss immediately after infection, then showed a better recovery trend in the following three days. The body weights of the hamsters in the VELDONA® Group remained more stable than those of the hamsters in the Placebo Group during the treatment period.
Mean Body Weight Throughout the Treatment Period
Percentage of Body Weight Change
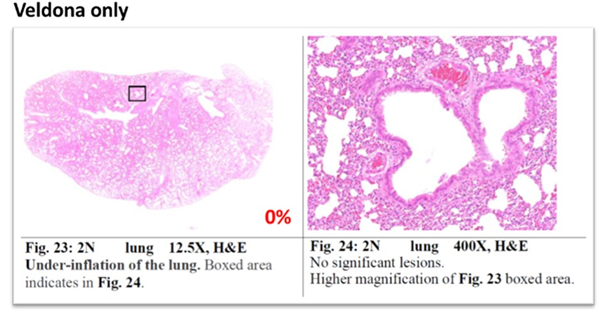
For pathological indicators, on the tenth day, no hamsters in the VELDONA® Group showed mixed-cellular inflammation, peribronchial infiltration, and perivascular infiltration, compared to 50% of hamsters in the Placebo Group. Hamsters in the VELDONA® Group in general showed promising results in treating indicators of new variant virus infection.
Lung Pathology Report
Mixed cellular inflammation, peribronchial infiltration, and perivascular infiltration.
Chun-Hsien Tsai, Ainos' Chairman of the Board, President, and Chief Executive Officer, commented, "Ainos has conducted three preclinical animal studies since March 2022 in our efforts to validate the effectiveness of our low-dose oral interferon to protect against symptoms associated with COVID-19 (Delta and Omicron variants). The studies have yielded consistent results, demonstrating VELDONA®'s efficacy in inducing systemic immunomodulatory to fight against SARS-COV-2. The results of the two-week study also showed that VELDONA® is well-tolerated and safe in hamsters. In addition, this new study exhibited a preventative and therapeutic effect against new variant virus. Ainos believe that these results, in combination with the results of our previous two Phase 2 studies of VELDONA® on prevention and treatment of influenza, demonstrate that VELDONA® may become an important solution for the treatment of COVID-19 and other viral infections in the future. Furthermore, we would like to thank the Emerging Infectious Disease Core Facility Platform of the National Defense Medical Center in Taiwan for the support and professional technical services they provided for the Study."
About Ainos, Inc.
Headquartered in San Diego, California, Ainos, Inc. (f/k/a Amarillo Biosciences, Inc.) is a diversified medtech company engaged in developing innovative medical technologies for point-of-care testing and safe and novel medical treatment for a broad range of disease indications. In addition to its proprietary therapeutics using low-dose non-injectable interferon, Ainos is committed to developing a comprehensive healthcare business portfolio encompassing medical devices and consumer healthcare products. While prioritizing the commercialization of medical devices as part of its diversification strategy, Ainos has also expanded its product portfolio to include Volatile Organic Compounds (VOC) and COVID-19 POCTs. Leveraging its patents related to VOC technologies and COVID-19 POCT products, the Company seeks to expedite the commercialization of its medical device pipeline, beginning with Ainos-branded COVID-19 POCT product candidates.
Forward-Looking Statements
This press release contains "forward-looking statements" about Ainos within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by the use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "project," "target," "future," "likely," "strategy," "foresee," "may," "guidance," "potential," "outlook," "forecast," "should," "will" or other similar words or phrases. Similarly, statements that describe the Company's objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements are based only on the Company's current beliefs, expectations, and assumptions. Forward-looking statements are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of the Company's control. The Company's actual results may differ materially from those indicated in the forward-looking statements.
Important factors that could cause the Company's actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release include, among others, the cost of production and sales potential of the planned drug treatments announced in this press release; the Company's dependence on revenues from the sale of COVID-19 test kits; the Company's limited cash and history of losses; the Company's ability to achieve profitability; the Company's ability to raise additional capital to continue the Company's product development; the ability to accurately predict the future operating results of the Company; the ability to advance Ainos' current or future product candidates through clinical trials, obtain marketing approval and ultimately commercialize any product candidates the Company develops; the ability to obtain and maintain regulatory approval of Ainos product candidates; delays in completing the development and commercialization of the Company's current and future product candidates, which could result in increased costs to the Company, delay or limit the ability to generate revenue and adversely affect the business, financial condition, results of operations and prospects of the Company; intense competition and rapidly advancing technology in the Company's industry that may outpace its technology; customer demand for the products and services the Company develops; the impact of competitive or alternative products, technologies and pricing; disruption in research and development facilities; lawsuits and other claims by third parties or investigations by various regulatory agencies governing the Company's operations; potential cybersecurity attacks; increased requirements and costs related to cybersecurity; the Company's ability to realize the benefits of third party licensing agreements; the Company's ability to obtain and maintain intellectual property protection for Ainos product candidates; compliance with applicable laws, regulations and tariffs; and the Company's success in managing the growth. A more complete description of these risk factors and others is included in the "Risk Factors" section of Ainos' most recent Annual Report on Form 10-K/A and other reports filed with the U.S. Securities and Exchange Commission, many of which risks are beyond the Company's control. In addition to the risks described above and in the Company's Form 10-K/A, other unknown or unpredictable factors also could cause actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release.
The forward-looking statements made in this press release are expressly qualified in their entirety by the foregoing cautionary statements. Ainos undertakes no obligation to, and expressly disclaims any such obligation to, publicly update or revise any forward-looking statement to reflect changed assumptions, the occurrence of anticipated or unanticipated events or changes to the future results over time or otherwise, except as required by law.
Investor Relations Contact
ICR, LLC
Robin Yang
Tel: +1 646-224-6971
Email: [email protected]
SOURCE: Ainos, Inc.



