KELOWNA, BC / ACCESSWIRE / December 21, 2022 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to announce it has demonstrated superior cannabidiol ("CBD") blood absorption levels from its patented DehydraTECH-CBD™ relative to those of published, pharmaceutical-grade CBD industry comparators in its recently completed, multi-week human clinical hypertension study HYPER-H21-4.
In a 2017 randomized clinical trial that evaluated a non-Lexaria, pharmaceutical-grade, plant-derived CBD formulation published by Devinski et. al., an average blood plasma CBD level of 23.0 ng/mL was evidenced after 22 days (so-called, "steady state"1) of daily dosing at a 5 mg/kg CBD dose level. By comparison, in Lexaria's 2022 HYPER-H21-4 hypertension study, a 45.8% higher average blood plasma level (33.3 mg/mL) was reached at DehydraTECH-CBD's lowest dose level tested of just 3.38 mg/kg, climbing to a blood plasma level that was 133.4% higher (53.7 ng/mL) at its highest dose level tested of 4.46 mg/kg.
The Devinsky publication also examined higher CBD dosing at a level of 10 mg/kg, evidencing a blood level of 62.1 ng/mL. Although Lexaria's HYPER-H12-4 study did not see the need to dose as high as 10 mg/kg, the data trend observed at its 3.38 and 4.46 mg/kg dose levels extrapolated linearly to 10 mg/kg would be expected to reach a level of 149.5 ng/mL; a 141% improvement over the Devinsky CBD formulation.
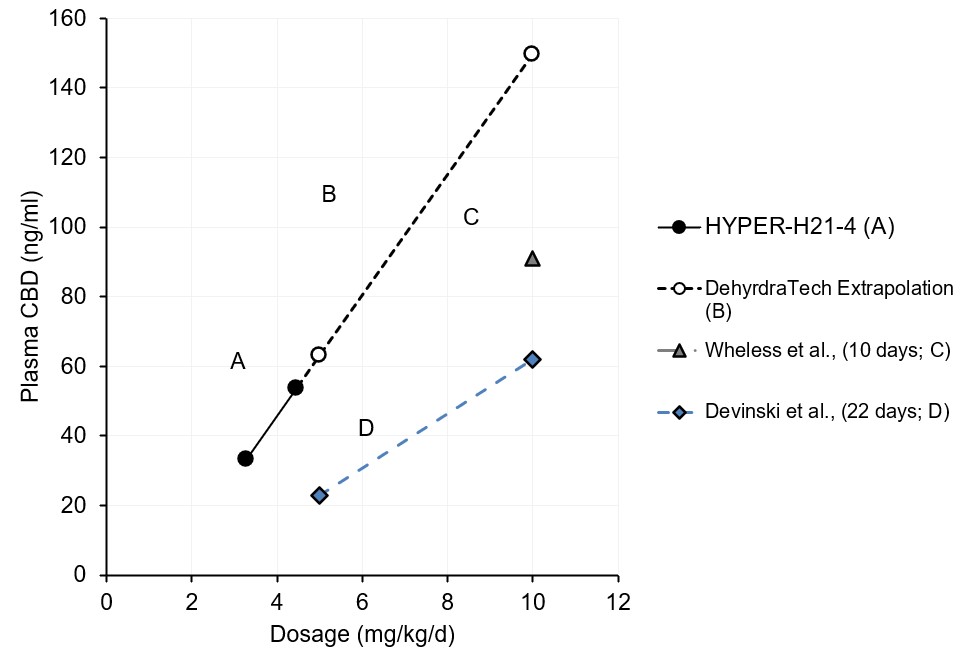
Similarly, a 2019 study published by Wheless et. al., also evaluated average CBD levels in blood following dosing to steady-state1 over a multi-day period, using a different pharmaceutical-grade, synthetic CBD formulation. At a 10 mg/kg dose level in the Wheless study, the non-Lexaria synthetic CBD reached a level within blood plasma of 91 ng/mL.; DehydraTECH-CBD absorption outperformed this synthetic CBD by 64% improvement upon linear extrapolation.
"DehydraTECH-CBD has repeatedly shown that it can be administered at much lower dose levels than other CBD formulations to achieve effective levels within the blood stream, as supported by this pharmaceutical-industry peer comparison," said Chris Bunka, CEO of Lexaria Bioscience Corp. "This is extremely important to patients hoping to achieve positive health outcomes while using lower levels of medication with no serious side effects and also important to Lexaria as we pursue FDA registration of DehydraTECH-CBD."
Additional study endpoint analyses as described in the complete study protocol are still underway and any relevant material findings will be reported upon in due course as these findings become available. It is highly likely that multiple additional datasets will be released in January through March of 2023.
Lexaria's HYPER-H21-4 study was a randomized, double-blinded, placebo-controlled, cross-over study of a total of 66 male and female volunteers between the ages of 40-70. All study participants received DehydraTECH-CBD every day for a total of 5 weeks. Blood CBD levels reported herein from DehydraTECH-CBD administration were measured prior to dosing, as was the case reported in the Devinsky article, whereas the Wheless article did not specify blood sampling timing relative to dosing. The complete HYPER-H21-4 study protocol has been published and is available at PubMed.
1Steady State
In Pharmacology, "steady-state" refers to the amount of time required before a consistent dose of drug achieves a stable blood plasma level. While the Devinsky and Wheless study references examined CBD levels in the blood at different points in time compared to Lexaria's study, all three studies would have been expected to have reached steady-state pharmacokinetics after sustained dose administration by the points in time at which sampling was performed. CBD is generally thought to reach steady-state dose levels within several days of consistent dosing.
ABOUT LEXARIA BIOSCIENCE CORP.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 27 patents granted and roughly 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. The Company only releases select, incomplete data from its study programs. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.




