In the Performance to Primary, Secondary and Tertiary Objectives second table the 90 day results were misstated and has been corrected in this press release.
TORONTO, ON / ACCESSWIRE / May 30, 2023 / Theralase® Technologies Inc. ("Theralase" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds ("PDCs") and their associated drug formulations intended to safely and effectively destroy various cancers has released the Company's 1Q2023 unaudited condensed interim consolidated Financial Statements ("Financial Statements").
Financial Summary:
For the three-month period ended March 31st:
Audited Consolidated Statements of Operations (In Canadian Dollars) | 2023 | 2022 | % Change | |||||||||
Revenue | ||||||||||||
Canada | 152,174 | 179,145 | -15 | % | ||||||||
United States | 55,037 | 32,517 | 69 | % | ||||||||
Total Revenue | 207,161 | 211,662 | -2 | % | ||||||||
Cost of Sales | 114,638 | 120,430 | -4 | % | ||||||||
Gross Margin | 92,523 | 91,232 | 1 | % | ||||||||
Gross Margin (% of revenue) | 45 | % | 43 | % | ||||||||
Operating Expenses | ||||||||||||
Selling Expenses | 74,671 | 87,640 | -15 | % | ||||||||
Administrative Expenses | 522,695 | 418,087 | 25 | % | ||||||||
Research and Development Expenses - CLT Division | 3,181 | 72,832 | -96 | % | ||||||||
Research and Development Expenses - ACT Division | 907,099 | 1,225,203 | -26 | % | ||||||||
Other 1 | (6,170 | ) | (11,041 | ) | -44 | % | ||||||
Total Operating Expenses | 1,501,476 | 1,792,721 | -16 | % | ||||||||
Net Loss | (1,408,953 | ) | (1,701,489 | ) | -17 | % | ||||||
1 Other represents foreign exchange, interest accretion on lease liabilities and / or interest income.
Financial Highlights:
Total revenue decreased 2%, year over year.
Cost of sales for the three-month period ended March 31, 2023 was $114,638 (55% of revenue) resulting in a gross margin of $92,523 (45% of revenue). In comparison, the cost of sales for the same period in 2022 was $120,430 (57% of revenue) resulting in a gross margin of $91,232 (43% of revenue). Cost of sales is represented by the following costs: raw materials, subcontracting, direct and indirect labour and the applicable share of manufacturing overhead. The gross margin increase, as a percentage of sales, year over year, is primarily attributed to a decrease in labour and material costs.
Selling expenses for the three-month period ended March 31, 2023 decreased to $74,671, from $87,640 for the same period in 2022 (15% decrease). The decrease in selling expenses is a result of reduced advertising (47%), and salaries (17%).
Administrative expenses for the three-month period ended March 31, 2023, increased to $522,695 from $418,087 for the same period in 2022 (25% increase). The increase in administrative expenses is primarily attributed to increased spending on general and administrative expenses (85%) and advisory fees (36%). Stock based compensation expense was higher in 2022 due to an increase in stock options granted.
Net research and development expenses for the three-month period ended March 31, 2023, decreased to $910,280 from $1,298,035 for the same period in 2022 (30% decrease). The decrease in research and development expenses for the three-month period is primarily attributed to the costs related to the manufacture of the Study II drug. Research and development expenses represented 77% of the Company's operating expenses and represent investment into the research and development of the Company's Anti-Cancer Therapy ("ACT") technology.
The net loss for the three-month period ended March 31, 2023, was $1,480,953, which included $244,787 of net non-cash expenses (i.e.: amortization, stock-based compensation expense and foreign exchange gain/loss). This compared to a net loss for the same period in 2022 of $1,701,489, which included $99,600 of net non-cash expenses. The ACT division represented $4,708,874 of this loss (82%) for the three-month period ended March 31, 2023. The decrease in net loss is primarily attributed to decreased spending on research and development expenses in Study II.
Operational Highlights:
1. Leadership Change
On May 24, 2023, Roger DuMoulin-White, B.Sc., P.Eng., Pro. Dir. was appointed President and Chief Executive Officer ("CEO") of the Company. Dr. Arkady Mandel, M.D., Ph.D., D.Sc. tendered his resignation as Interim CEO and continues to serve as Chief Scientific Officer ("CSO") and as a member of Theralase®'s Board.
Mr. DuMoulin-White is the founder of Theralase® and it's former President and CEO. He stepped down as President and CEO in 2018 and has since served in a non-executive business development role.
Mr. DuMoulin-White was the subject of a voluntary Settlement Agreement with the Ontario Securities Commission ("OSC") dated February 16, 2018 and an OSC Order dated February 26, 2018, which required, among other things, that he resign as a director and officer of Theralase® and refrain from holding those positions for a period of five years. That period has expired and Theralase® has obtained the approval of the Toronto Stock Venture Exchange ("TSXV") to appoint Mr. DuMoulin-White as President and CEO of the Company and to nominate him for election to the Company's Board of Directors at the Company's Annual Meeting on June 29, 2023.
2. TSX Venture 50TM
Theralase® was named to the Toronto Stock Exchange Venture ("TSXV") "2023 Venture 50™". The Venture 50™ is an annual ranking of the top-performing companies from five industry sectors; specifically: Clean Technology and Life Sciences, Diversified Industries, Energy, Mining and Technology. Theralase® was recognized in the Clean Technology and Life Sciences category. Theralase® was previously named a 2015, 2019 and 2020 Venture 50™ company making this the fourth year Theralase® has been recognized as a top performer in the Clean Technology and Life Sciences sector in the last 8 years.
3. Warrant Extension
On January 5, 2023, the Company extended the expiry date of 4,095,157 share purchase warrants, all of which are exercisable at $0.50 per share. The share purchase warrants were issued on January 9, 2019 pursuant to a private placement involving the issuance of 4,095,157 units of the Company. The new expiry date of the warrants is January 9, 2024.
4. Study II Update
To date, Study II has provided the primary study treatment for 59 patients.
In recent discussions with the Medical and Scientific Advisory Board ("MSAB") for Study II, the MSAB advised the Company to review the FDA Guidance to Industry1 on how to best classify Indeterminate Response ("IR") patients (patients assessed with negative cystoscopy and positive urine cytology), where the source of the positive urine cytology has not been determined.
The FDA Guidance to Industry2 states as follow:
"For single-arm trials of patients with BCG-unresponsive disease, the FDA defines a complete response as at least one of the following:
- Negative cystoscopy and negative (including atypical) urine cytology
- Positive cystoscopy with biopsy-proven benign or low-grade NMIBC and negative cytology
For intravesical therapies without systemic toxicity, the FDA includes, in the definition of a complete response, negative cystoscopy with malignant urine cytology if cancer is found in the upper tract or prostatic urethra and random bladder biopsies are negative."
Theralase®'s Study II treats patients with an intravesical study drug activated by an intravesical study device. In accordance with the FDA Guidance to Industry, patients enrolled and provided the primary study treatment, where the source of the positive urine cytology has not been identified (i.e.: upper tract or prostatic urethra Urothelial Cell Carcinoma ("UCC")) and confirmatory bladder biopsies were negative, Theralase® has reclassified these patients from Indeterminate Response ("IR") to Complete Response ("CR").
For patients, who have been enrolled and provided the primary study treatment in Study II, that have been diagnosed as IR and do not have confirmatory negative bladder biopsies (confirming that the source of the UCC is not from the bladder wall), these patients have remained classified as IR, until additional clinical assessments can be completed by the PIs to prove or disprove a diagnosis of CR.
As a result, Theralase® updated its Study II's interim clinical study data analysis, where some patients have been reclassified from IR to CR on certain assessment days.
In accordance with the FDA Guidance to Industry, Theralase® will conduct sensitivity analyses, in which these IR patients are considered not to have achieved a CR, as a part of the final clinical report.
In 2016, Kamat et al. stated in the Journal of Clinical Oncology that the International Bladder Cancer Group ("IBCG") recommended that, "Single-arm designs may be relevant for the BCG-unresponsive population. Here, a clinically meaningful initial complete response rate (for carcinoma in situ) or recurrence-free rate (for papillary tumors) of at least 50% at 6 months, 30% at 12 months, and 25% at 18 months is recommended."2
The interim clinical data presented below meets or exceeds these IBCG guidelines.
Performance to Primary, Secondary and Tertiary Objectives:
Assessment | Achieved Primary Objective | Achieved Secondary Objective | Achieved Tertiary Objective |
Complete Response ("CR") | 66% | 33% | 100% |
Indeterminate Response ("IR") | 8% | 5% | - |
Total Response ("CR + IR") | 74% | 38% | 100% |
Evaluable Patients | 53 | 39 | 53 |
In an analysis of Evaluable Patients, Study II clinical data provides the following interim analysis:
Assessment | 90 Day | 180 Day | 270 Day | 360 Day | 450 Day |
Complete Response ("CR") | 60% | 53% | 43% | 36% | 33% |
Indeterminate Response ("IR") | 6% | 18% | 12% | - | 5% |
Total Response ("CR + IR") | 66% | 71% | 55% | 36% | 38% |
Evaluable Patients | 53 | 49 | 42 | 39 | 39 |
The interim clinical data demonstrates that 60% of Evaluable Patients (Patients evaluated by a Principal Investigator ("PI") achieved a CR at 90 days post primary Study Treatment and 33% achieved a CR at 450 days.
Note:
- For patients to be included in the statistical clinical analysis, they must be enrolled in Study II, provided the primary Study Treatment and evaluated by a PI at the 90 day assessment visit (cystoscopy and urine cytology)
- One patient passed away prior to their 90 day assessment and is therefore not included in the statistical analysis.
- Evaluable Patients are defined as patients who have been evaluated by a PI and thus excludes a patient's clinical data at specific assessment days, if that clinical data is pending.
- Five patients have been enrolled and provided the primary Study Treatment, but have not been evaluated at their 90 day assessment; therefore, 53 patients are considered Evaluable Patients at 90 days, with 39 patients considered Evaluable Patients at 450 days.
- The data analysis presented above, should be read with caution, as the clinical data is interim in its presentation, as Study II is ongoing and new clinical data collected may or may not continue to support the current trends, with significant data still pending.
- Total Responders (CR and IR) are defined as CR + IR.
- For patients who have been removed from the study by the PI or have elected to discontinue from the clinical study their last observation has been carried forward in this analysis.
The Swimmer's plot in Figure 1.0 is a graphical representation of the interim clinical results (n=58) showing a patient's response to a treatment over time. As can be seen in the plot, significant data is still pending.
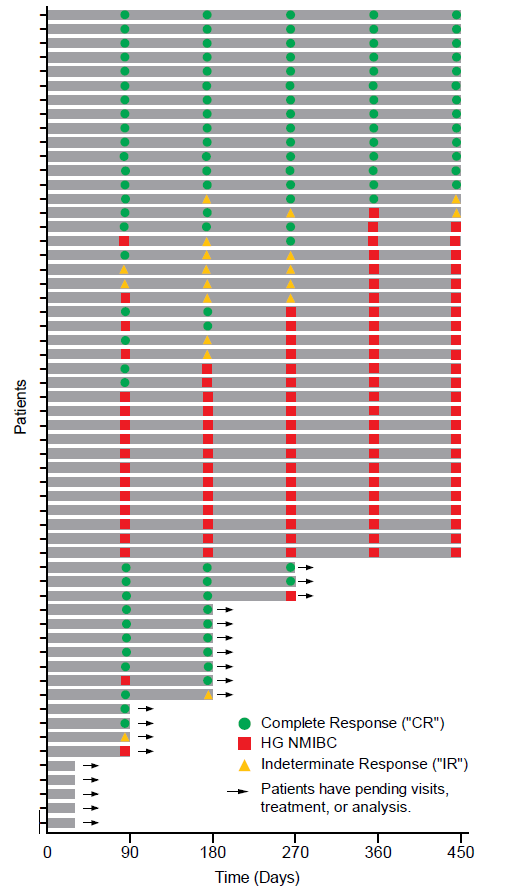
Figure 1.0: Swimmer's Plot
The Swimmer's Plot illustrates:
- 13 Evaluable Patients that achieved CR at each assessment date and thus achieved the primary and secondary objectives of Study II for all patients assessed up to 450 days (13/39 = 33%).
- 35 Evaluable Patients that achieved CR on at least one assessment date and thus achieved the primary objective of Study II (35/53 = 66%)
The interim Kaplan-Meier ("KM") Curve (Figure 2.0) represents the cumulative incidence of clinical events, including the treatment efficacy, occurring over a prespecified time in Study II. According to the KM curve, approximately 80% of patients remained in Study II after 90 days, following the primary study treatment. More than 60% of the treated patients have a probability to achieve the primary study objective and 34% of patients have a probability to achieve a durable CR (the Study II secondary endpoint) at 450 days.
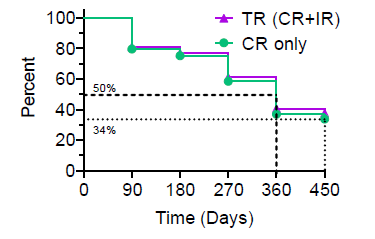
Figure 2.0: Kaplan-Meier ("KM") Curve
5. Study II Interim Data Presentations
Study II (Interim) clinical data was presented at the American Society of Clinical Oncology ("ASCO") Genito Urinary ("GU") Cancer Symposium on February 17, 2023 in San Francisco, California with the poster presented for general viewing and discussion within Poster Session B: Prostate Cancer and Urothelial Carcinoma. The poster presented at the ASCO GU Cancer Symposium can be found on the Company's website at www.theralase.com/ASCO_Poster.
Study II (Interim) clinical data was presented at the 2023 American Urology Association ("AUA") Annual Meeting on May 1st, 2023 in Chicago, Illinois with the poster presented for general viewing and discussion at a moderated poster session, within the AUA Annual Meeting. The poster presented at the AUA Annual Meeting can be found on the Company's website at www.theralase.com/AUA_Poster.
2 BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment - Guidance for Industry. February 2018; www.fda.gov/media/101468/download
3 Kamat AM et al. J Clin Oncol. 2016; 34: 1935-1944
About Study II
Study II utilizes the therapeutic dose of TLD-1433 (0.70 mg/cm2) activated by the proprietary TLC-3200 medical laser system. Study II is focused on enrolling and treating approximately 100 to 125 BCG-Unresponsive Non-Muscle Invasive Bladder Cancer ("NMIBC") Carcinoma In-Situ ("CIS") patients in up to 15 Clinical Study Sites ("CSS") located in Canada and the United States.
Study II objectives are as follows:
Primary:
Efficacy - evaluated by CR at any point in time patients diagnosed with CIS (with or without resected papillary Ta/T1 disease)
CR is defined as at least one of the following:
- Negative cystoscopy and negative (including atypical) urine cytology
- Positive cystoscopy with biopsy-proven benign or low-grade NMIBC and negative cytology
- Negative cystoscopy with malignant urine cytology, if urothelial cancer is present in the upper tract or prostatic urethra and random bladder biopsies are negative
Secondary:
Duration of CR - evaluated as sustainability of CR at 12 months post initial CR.
Tertiary:
Safety - evaluated by the incidence and severity of Adverse Events ("AEs") directly related to the Study Drug and/or Study Device, Grade 4 or higher that do not resolve within 450 days post treatment (where: Grade 1 = Mild, Grade 2 = Moderate, Grade 3 = Severe, Grade 4 = Life-threatening or disabling, Grade 5 = Death)
About TLD-1433 (RuvidarTM)
TLD-1433 is a patented PDC with 12 years of published peer reviewed preclinical research and is currently under investigation in Study II.
The trade name RuvidarTM was selected by the Company, as Ru is the element symbol for Ruthenium, a rare transition metal belonging to the platinum group, which the Theralase ® PDC is based upon, vita is Latin for life and dar is Russian for gift; hence, roughly translated, "Ruthenium, the gift of life".
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated compounds and their associated drug formulations with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Forward looking statements may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's Photo Dynamic Compounds and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to: adequately fund, and secure the requisite regulatory approvals to successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available or may not be available on terms that are commercially favorable to the Company, the risk that the Company's drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the term of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings, and the impacts of public health crises, such as COVID-19. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward- looking statements which are not a guarantee of future performance. There can be no assurance that forward looking statements will prove to be accurate as such forward looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey CPA, Chief Financial Officer
[email protected]
SOURCE: Theralase Technologies Inc.




