- DMT310 once-weekly topical treatment demonstrated statistically significant improvements at all time points in the three primary endpoints, inflammatory lesion count, noninflammatory lesion count, and Investigator Global Assessment (IGA) -
- DMT310 significantly reduced inflammatory lesions by 45% after only 4 treatments in patients with moderate-to-severe acne -
- FDA responses to DMT310 End of Phase 2 meeting package expected by the end this month -
SAN DIEGO, CA / ACCESSWIRE / June 8, 2023 / Dermata Therapeutics, Inc. (Nasdaq:DRMA, DRMAW) ("Dermata" or the "Company"), a clinical-stage biotechnology company focusing on the treatment of medical and aesthetic skin conditions, today announced that positive results from its DMT310 Phase 2b study of its once-weekly topical treatment in patients with moderate-to-severe acne vulgaris was published in the prestigious, peer-reviewed Journal of the American Academy of Dermatology (JAAD) and can by found at this link. The study found that once-weekly topical treatment with DMT310 resulted in significant improvements across multiple efficacy endpoints at 12 weeks in patients with moderate-to-severe acne when compared with placebo.
"The publication of our DMT310 Phase 2b results in a journal as prominent as JAAD is a validation of the positive results generated by our lead asset for the potential treatment of acne," said Gerry Proehl, Dermata's Chairman, President, and Chief Executive Officer. "We continue to be excited by the Phase 2b data that we believe clearly highlights DMT310's ability to address the many challenges with current acne therapies. DMT310's once-weekly application is less burdensome than once or twice a day topical therapies, has a rapid time to treatment effect, and appears to be safe and well tolerated. We believe these attributes, combined with a significant treatment effect, could position DMT310 as a first line treatment for patients with moderate-to-severe acne while also being able to improve patient compliance. We look forward to receiving feedback from FDA on our End of Phase 2 meeting package by the end of June 2023. Assuming positive feedback, we plan to quickly initiate our DMT310 Phase 3 program in moderate-to-severe acne."
DMT310 Phase 2b Trial Design
The DMT310 Phase 2b randomized, double-blind, placebo-controlled trial was designed to evaluate the efficacy, safety, and tolerability of DMT310 applied once-weekly in 181 participants 12 years of age and older with moderate-to-severe facial acne. Participants were randomized in a 1:1 ratio to receive either DMT310 mixed with 6 ml of 3% hydrogen peroxide or placebo mixed with 6 ml of 3% hydrogen peroxide. The assigned study drug was applied to the entire face once-weekly for 12 weeks.
DMT310 Phase 2b Key Findings
- All primary and secondary efficacy endpoints were met.
- DMT310 demonstrated statistically significant reductions in inflammatory (-15.6 vs. -10.8, p < 0.01) and noninflammatory (-18.3 vs. -12.4, p < 0.01) lesion counts at week 12 when compared to placebo.
- Notably, DMT310 patients experienced an early statistically significant effect in the percent reductions from baseline in inflammatory (-45.2% vs. -23.8%, p < 0.001) and noninflammatory (-36.4% vs. -14.3%, p < 0.001) lesion counts at week 4 when compared to placebo.
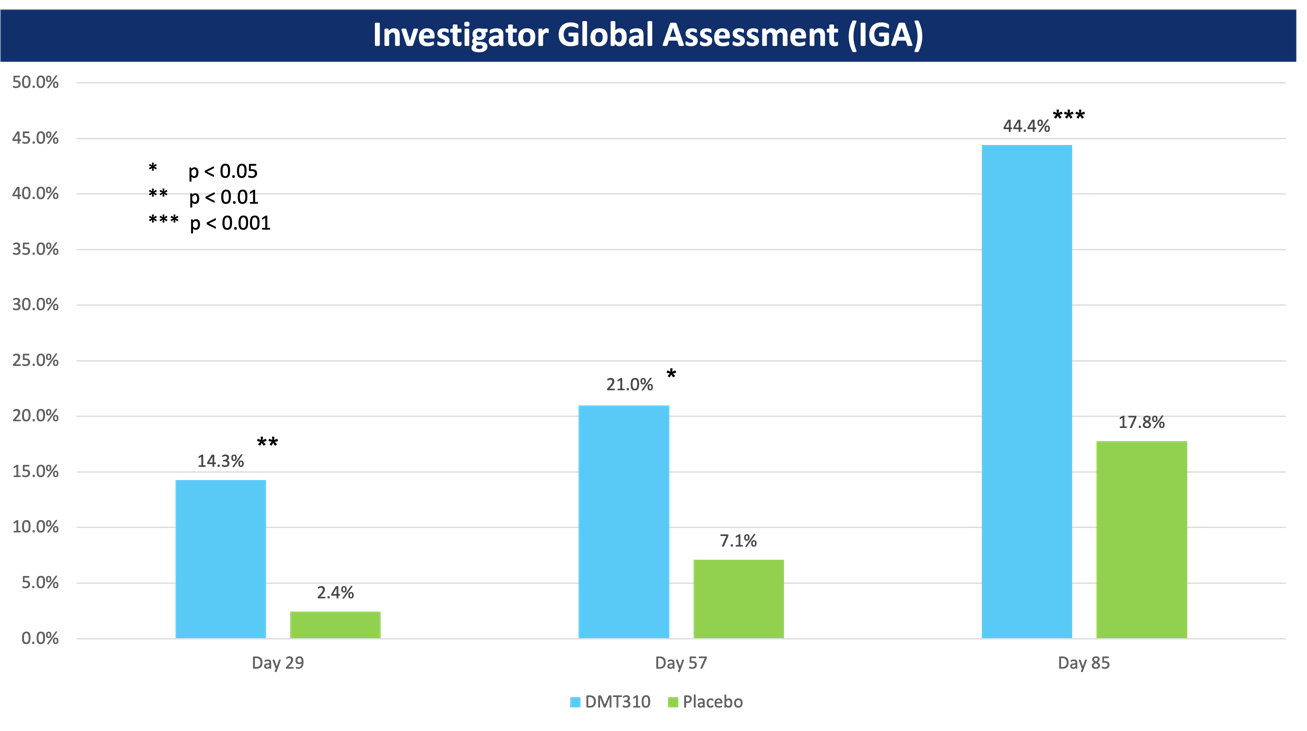
- DMT310 also demonstrated statistically significant improvements in IGA at all time points with a responder defined as having a 2-grade change and an IGA score of 0 (clear) or 1 (almost clear), as seen in the image below.
DMT310 appeared to be safe and well tolerated with no drug-related serious adverse events reported during the 12-week study.
About Acne Vulgaris
Acne affects approximately 50 million people in the U.S., with about 85% of teenagers experiencing some form of acne, and some individuals suffering from acne well into their 30s, 40s, and beyond. Acne is characterized by areas of scaly red skin, noninflammatory blackheads and whiteheads, inflammatory papules and pustules, and occasionally cysts and scarring, which occurs on the face, neck, chest, back, shoulders, and upper arms. While not life-threatening, acne can cause significant trauma for those suffering from it due to social stigmas, substantial risk of permanent facial scarring, lowered self-esteem, and social withdrawal.
About Dermata Therapeutics
Dermata Therapeutics, Inc. is a clinical-stage biotechnology company focusing on the treatment of medical and aesthetic skin conditions. The Company's lead product candidate, DMT310, is the first product candidate being developed from its Spongilla technology platform. DMT310 is a once-weekly topical product candidate derived from a naturally sourced freshwater sponge with multiple unique mechanisms of action. In addition to acne, DMT310 has been studied for the treatment of psoriasis and rosacea. The Company's second product candidate, DMT410, uses its Spongilla technology as a new method for needle-free intradermal delivery of botulinum toxin for the treatment of multiple aesthetic and medical skin conditions. Dermata is headquartered in San Diego, California. For more information, please visit http://www.dermatarx.com/.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are based on the Company's current beliefs and expectations and new risks may emerge from time to time. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but are not limited to, statements related to: expectations with regard to the potential market acceptance of any of the Company's product candidates; timing of data events; expectations with regard to the timing and/or results from meetings with regulatory bodies; the Company's expectations with regard to current cash and the amount of time it will fund operations; the success, cost, and timing of its product candidate DMT310 development activities and ongoing and planned clinical trials; and whether the results of DMT310 will lead to future product development or approvals. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks and uncertainties inherent in drug development, approval and commercialization, and the fact that past results of clinical trials may not be indicative of future trial results. For a discussion of these and other factors, please refer to Dermata's filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All forward-looking statements are qualified in their entirety by this cautionary statement and Dermata undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof, except as required by law.
Investors:
Sean Proehl
Senior Director, Legal and Business Development
[email protected]
SOURCE: Dermata Therapeutics




