- Blood glucose levels reduced by 19.9% with DehydraTECH-CBD
- Improvements in kidney function also demonstrated
KELOWNA, BC / ACCESSWIRE / June 16, 2023 / Lexaria Bioscience Corp. (Nasdaq:LEXX)(Nasdaq:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to report upon additional findings from its diabetes animal study DIAB-A22-1, and also provide updates on two other applied R&D programs.
Diabetes: DIAB-A22-1
As announced on March 2, 2023, Lexaria completed initial testing using DehydraTECH-CBD in its diabetes animal model study that produced three positive outcomes including weight loss in obese diabetic-conditioned animals, together with improved triglyceride and cholesterol levels. Following this, Lexaria undertook a further round of analysis to explore additional study outcomes, including the use of an alternate blood glucose assay detection system with higher detection sensitivity than was used in the initial testing round.
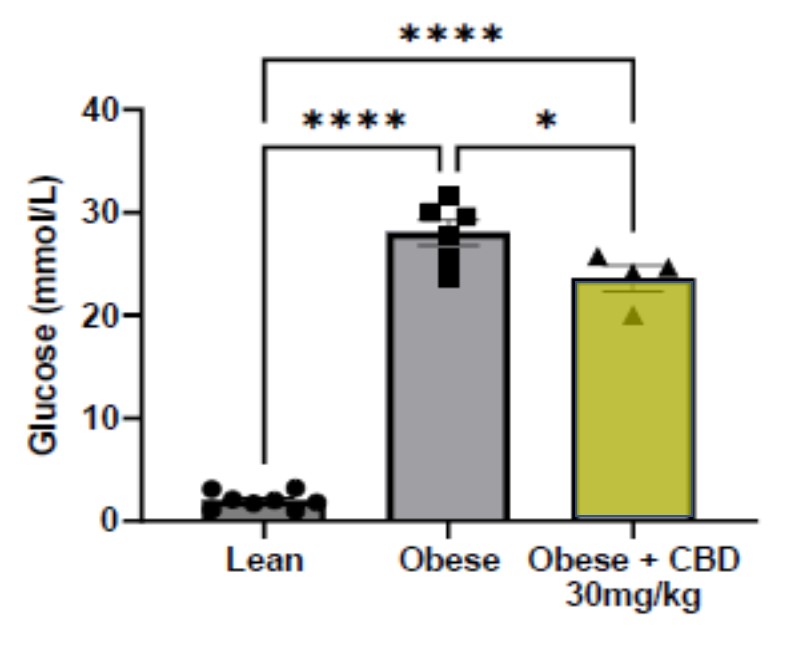
Using the Antech hexokinase blood chemistry test panel methodology, Lexaria discovered that blood glucose levels were statistically significantly lowered by 19.9 ± 7% in the obese diabetic-conditioned animals treated with the DehydraTECH-CBD 30 mg/Kg dose (yellow bar above) (*p<0.05) compared to the obese vehicle control animals. This appears to be a new discovery of a property not generally known to be associated with generic CBD treatment.
Kidney function was also evaluated compared to the vehicle control animals by examination of the levels of blood urea nitrogen ("BUN"), creatinine, and assessment of the BUN/creatinine ratio. BUN levels were reduced by 27.9% +/- 5% (***p<0.001) in the obese animals receiving DehydraTECH-CBD. Creatinine levels were also improved with a 16.8% +/-7% increase in the obese animals receiving DehydraTECH-CBD, although this improvement was not statistically significant.
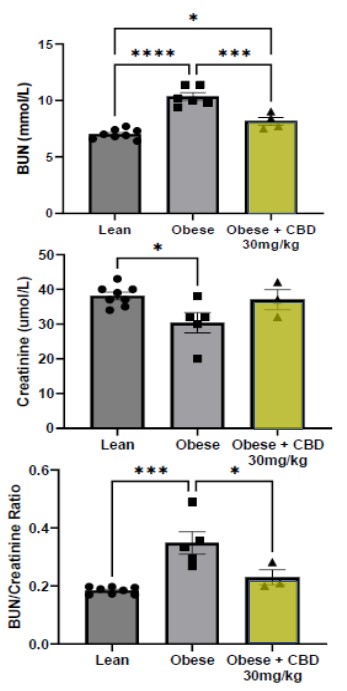
Astonishingly, the calculated BUN/creatinine ratio in the obese animals being treated with DehydraTECH-CBD returned to a healthy range nearly equal to that of the lean animals, with a 55.1% +/-16% reduction (*p<0.05) (yellow bars above indicate DehydraTECH-CBD treatment). This may reflect an effect of DehydraTECH-CBD to correct abnormal kidney function in this animal model as another prospective benefit in treating pathological conditions commonly associated with diabetes.
DehydraTECH-CBD's ability to reduce blood sugar levels in animals is extremely encouraging and certainly warrants additional investigation. According to the Center for Disease Control, managing your blood sugar levels is important to avoid diabetes-related conditions such as vision loss, heart disease, and kidney disease. Generic CBD has not, in general, been found to be effective in controlling blood sugars and the Company has not yet discovered whether DehydraTECH-CBD might be effective in blood-sugar control in humans.
Dementia: DEM-A22-1
As announced on November 10, 2022, study DEM-A22-1 was undertaken in thirty-two Long Evans rats using the established novel object recognition test ("NOR"), which is used to assess memory in rodents, in order to investigate whether DehydraTECH-CBD enables any pro-cognitive performance enhancements in this model, potentially of use in dementia treatment.
The findings from this study were generally unremarkable and several unexpected study complications may have muted efficacy distinction ability. Most notably, the aged rats where cognitive impairment was expected to be most obvious did not, in fact, adequately exhibit this impaired tendency, making "improvements" difficult to measure. Also, animals in the high dose DehydraTECH-CBD cohort exhibited reduced locomotor activity, which may have indicated that treatment at this level produced sedative activity, also masking any prospective cognitive improvement. Together, these inconclusive results may point to required study design improvements necessary for a more concrete set of results.
While this research did not produce any notable outcomes, Lexaria now recognizes that previous research by other investigators using CBD in this animal model has required dosing over a much longer duration (over a period of several weeks in order to demonstrate NOR enhancement), than Lexaria has thus far evaluated-. Lexaria will consider this if further testing is considered in future, potentially also including DehydraTECH-processed nicotine, as previously announced, as another agent known to enhance cognitive performance when effectively delivered.
Epilepsy: EPIL-A21-1
As announced on November 29, 2022, Lexaria successfully completed two parts of its EPIL-A21-1 study program designed to evaluate the effectiveness of its DehydraTECH-CBD compared to one of the world's leading anti-seizure medications, Epidiolex®, in reducing seizure activity using an established, vehicle-controlled, acute animal seizure model induced by electrical stimulation ("MES"). The findings from this work appear to demonstrate that DehydraTECH-CBD had effectiveness at lower doses and with greater rapidity than Epidiolex®.
Since that time, Lexaria has also completed a final MES study under its EPIL-A21-1 program designed to establish an ED50 (i.e., the dose required to achieve seizure inhibition in 50% of the animals tested) for DehydraTECH-CBD in this animal model, where ED50 determination is a common performance metric in preclinical animal studies for developmental therapeutics. This ED50 study was designed with an objective to hopefully corroborate Lexaria's prior MES experimental findings. Lexaria is pleased to confirm that the outcome demonstrated that DehydraTECH-CBD was most effective at a dose of 75 mg/Kg, also as previously reported from the initial round of work in this animal model which compared favourably to Epidiolex® that generally required a higher dose of 100 mg/Kg to achieve comparable findings.
About DehydraTECH
DehydraTECH is a patented drug delivery formulation and processing platform technology Lexaria has developed and is investigating for a variety of beneficial molecules. DehydraTECH is designed to improve the way active molecules enter the bloodstream upon oral ingestion. DehydraTECH has also demonstrated enhanced delivery of certain active molecules into brain tissue, which Lexaria believes to be of particular importance for centrally active compounds. Lexaria has also developed DehydraTECH formulations for other applications demonstrating superior bio-absorption when administered intraorally and topically.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 30 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
[email protected]
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.




