Ainos' AI-Powered Digital Nose Technology to Transform Telehealth and Point-of-Care Testing
Clinical Trials of AI Nose-powered POCT to be Completed by the End of 2023, Positioning Ainos to Capitalize on US$256 Billion STI Testing Market
SAN DIEGO, CA / ACCESSWIRE / June 20, 2023 / Ainos, Inc. (NASDAQ: AIMD, AIMDW) ("Ainos", or the "Company"), a diversified medtech company focused on the development of novel point-of-care testing, low-dose interferon therapeutics, and synthetic RNA-driven preventative medicine, provides insights into its transformative AI-powered digital nose technology, AI Nose, including its design, functionality, product differentiation, and disruptive potential in the telehealth industry.
Having garnered over 50 active and pending patents, Ainos' AI Nose technology embodies the essence of over 10 years of research and development in digital nose sensing, telehealth, and point-of-care testing. It seamlessly integrates artificial intelligence with digital nose sensors, and serves as the core growth engine for Ainos, whose company name is a fusion of the words "AI" and "Nose". With advances in chip computing power and memory capacity, Ainos has utilized deep learning and other advanced AI algorithms to accelerate productization of its AI Nose technology by leaps and bounds.
Ainos' AI Nose consists of an array of digital nose sensors, which are primarily used to detect volatile organic compounds (VOCs). The VOC signals collected by these sensors undergo pre-processing, followed by feature selection extracting data on each signal's unique characteristics. Leveraging deep neural networks, clinical trial data labeling, and supervised machine learning, Ainos' AI Nose can accurately identify VOC samples.
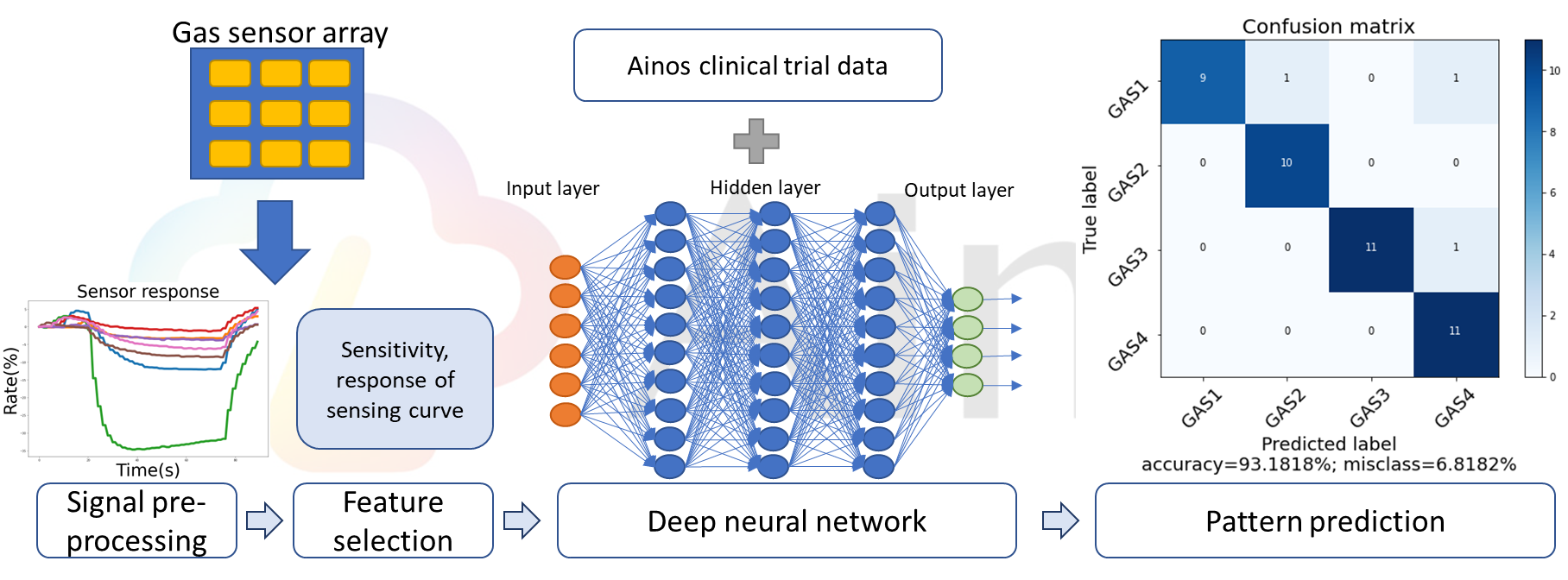
Figure 1: AI Nose Algorithm
The human body routinely emits hundreds of trace gases, or VOCs, through respiration, perspiration, and other bodily functions. These VOCs carry valuable physiological information and often correlate with the presence of specific diseases, meaning they can be used to diagnose certain medical conditions. Ainos leverages its AI digital nose technology to detect VOCs associated with human metabolic processes, and is currently developing a series of non-invasive VOC point-of-care testing (POCT) devices.
The COVID-19 pandemic accelerated the adoption of telehealth solutions, and remote-enabled POCT devices represent a vital part of telehealth's future. Ainos' AI Nose-powered rapid tests offer users convenience, speed, and simplicity, making them an ideal POCT solution for hospitals, communities, and home-based healthcare.
The Company's flagship VOC POCT device, Ainos Flora, was the 2021 recipient of iF's Medicine/Health Design Award. Ainos Flora is designed to quickly and discreetly detect bacteria, fungi, and sexually transmitted infections in women. The compact, non-invasive device collects gas samples in the vicinity of the patient's genitalia without requiring direct bodily contact. This fast and private testing experience aids doctors in swiftly diagnosing and treating patients, while the device's portability and ease of use enables individuals to remotely self-monitor and actively manage their personal health.
Ainos is currently conducting clinical trials of Ainos Flora in four major medical centers in Taiwan. These trials involve the collection of massive amounts of metabolic gas data, which is used to train highly accurate AI algorithms. Clinical research validation for Ainos Flora is projected to be complete by the end of 2023, paving the way for certification, market approval, and eventual commercialization.
The future of healthcare lies in the integration of AI-enabled medical devices, telehealth services, and cloud applications. Ainos' medical devices generate significant amounts of data during the testing process (see Figure 2), and big data analysis of this information constantly renders algorithms more accurate. Meanwhile, long-term monitoring of individual health data in combination with these algorithms and cloud databases will help detect diseases early on and aid in their prevention. As a result, patients will be able to achieve a level of precision health that has historically been impossible.

Figure 2: Role of AI Nose in Telehealth
Chun-Hsien (Eddy) Tsai, Chairman of the Board, President, and CEO of Ainos, commented, "The role of AI in medicine will only grow more important as technology progresses. As it does, Ainos will maintain its AI expertise and lead the charge in smart medical device development. Our innovative AI Nose technology, and the POCT solutions which utilize it, perfectly suit the proliferation of telehealth solutions we have seen in COVID's aftermath. By leveraging our extensive experience and our unrelenting desire for progress, I am confident that Ainos will become an integral part of the telehealth-focused, AI-enabled future of healthcare. Furthermore, I believe that Ainos Flora, with its ease of use, is well-positioned to address the growing STI testing market, which is projected to reach US$256 billion by 2028."
About Ainos, Inc.
Headquartered in San Diego, California, Ainos, Inc. is a diversified medtech company engaged in developing innovative medical technologies for point-of-care testing and safe and novel medical treatment for a broad range of disease indications. In addition to its proprietary therapeutics using low-dose non-injectable interferon, Ainos has also expanded its product portfolio to include Volatile Organic Compounds (VOC) and COVID-19 POCTs. Powered by its AI Nose platform, the lead POCT candidate, Ainos Flora, is a telehealth-friendly POCT for women's health and certain common STIs. To learn more, visit https://www.ainos.com.
Follow Ainos on Twitter (@AinosInc) and LinkedIn to stay up-to-date.
Forward-Looking Statements
This press release contains "forward-looking statements" about Ainos within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by the use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict," "project," "target," "future," "likely," "strategy," "foresee," "may," "guidance," "potential," "outlook," "forecast," "should," "will" or other similar words or phrases. Similarly, statements that describe the Company's objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements are based only on the Company's current beliefs, expectations, and assumptions. Forward-looking statements are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of the Company's control. The Company's actual results may differ materially from those indicated in the forward-looking statements.
Important factors that could cause the Company's actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release include, among others, the cost of production and sales potential of the planned drug treatments announced in this press release; the Company's dependence on revenues from the sale of COVID-19 test kits; the Company's limited cash and history of losses; the Company's ability to achieve profitability; the Company's ability to raise additional capital to continue the Company's product development; the ability to accurately predict the future operating results of the Company; the ability to advance Ainos' current or future product candidates through clinical trials, obtain marketing approval and ultimately commercialize any product candidates the Company develops; the ability to obtain and maintain regulatory approval of Ainos product candidates; delays in completing the development and commercialization of the Company's current and future product candidates, which could result in increased costs to the Company, delay or limit the ability to generate revenue and adversely affect the business, financial condition, results of operations and prospects of the Company; intense competition and rapidly advancing technology in the Company's industry that may outpace its technology; customer demand for the products and services the Company develops; the impact of competitive or alternative products, technologies and pricing; disruption in research and development facilities; lawsuits and other claims by third parties or investigations by various regulatory agencies governing the Company's operations; potential cybersecurity attacks; increased requirements and costs related to cybersecurity; the Company's ability to realize the benefits of third party licensing agreements; the Company's ability to obtain and maintain intellectual property protection for Ainos product candidates; compliance with applicable laws, regulations and tariffs; and the Company's success in managing the growth. A more complete description of these risk factors and others is included in the "Risk Factors" section of Ainos' most recent Annual Report on Form 10-K and other reports filed with the U.S. Securities and Exchange Commission, many of which risks are beyond the Company's control. In addition to the risks described above and in the Company's Form 10-K, other unknown or unpredictable factors also could cause actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release.
The forward-looking statements made in this press release are expressly qualified in their entirety by the foregoing cautionary statements. Ainos undertakes no obligation to, and expressly disclaims any such obligation to, publicly update or revise any forward-looking statement to reflect changed assumptions, the occurrence of anticipated or unanticipated events or changes to the future results over time or otherwise, except as required by law.
Investor Relations Contact
ICR, LLC
Robin Yang
Tel: +1 646-224-6971
Email: [email protected]



