Preventogen's FDA-Cleared Microbicidal Liquid Polymer Ensures the Eradication of These Dangerous Pathogens on Contact While Forming a More Effective and Measurable Barrier to Optimize Healing
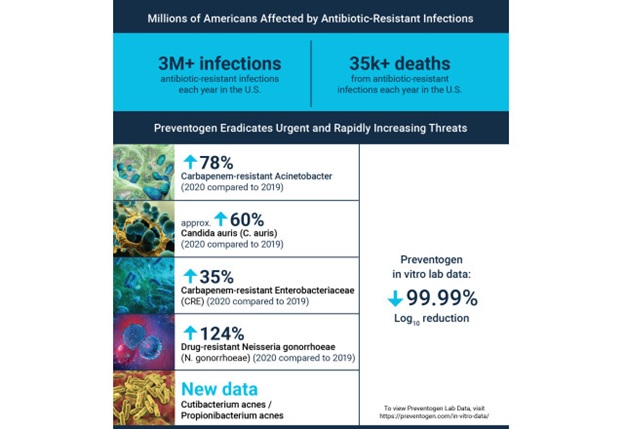
ST. LOUIS, MO / ACCESSWIRE / December 12, 2023 // Many may not know the immense problem of antimicrobial resistance. A routine surgery or common cut can quickly become life-threatening due to an infection; an otherwise healthy person can quickly become fatally ill. These superbugs have already affected millions of Americans, and the problem of antimicrobial resistance is accelerating in our post-COVID world.

Preventogen Logo
SEEKING A SOLUTION
In 2019, the Centers for Disease Control (CDC) in Atlanta published a 180-page compendium of the deadliest antibiotic-resistant threats: "Antibiotic resistance threats in the United States, 2019". This groundbreaking work highlighted the 18 most dangerous pathogens and classified them as Urgent, Serious, or Concerning threats.
In its most recent publication, "COVID-19 Reverses Progress in Fight Against Antimicrobial Resistance in the U.S.", the CDC cited a rise of 15% in hospitalization-related infections from 2019 to 2020. These pathogens are becoming stronger and more resistant on a global scale. In a January 2022 report, antimicrobial resistance was shown to be a leading cause of death globally, with the most significant burden in low-resource countries.
A SOLUTION WITH ROBUST KILLING PROPERTIES
Prevent-Plus has a solution in Preventogen™, a non-antibiotic approach to infection prevention. Since its inception in 2016, Prevent-Plus has performed in-vitro and flesh pad laboratory testing. After reading the 2019 CDC report, those most dangerous pathogens became vital targets.
Preventogen's effectiveness has been definitively shown against every pathogen it has been tested against. All testing has been completed at Nelson Labs®, a Sotera Health company and an industry-leading, global provider of laboratory testing, performing over 900 rigorous microbiological and analytical laboratory tests.
"Our company is dedicated to the prevention of infection, which is why we continue to collect evidence to demonstrate Preventogen's safety and efficacy in both lab and clinical settings," said Brad Chartrand, CEO of Prevent-Plus.
Prevention is an FDA-cleared topical liquid polymer that kills bacteria, viruses, and fungi on contact. When applied to the skin, the patented polymer dries quickly. It creates a non-antibiotic, elastomeric barrier designed to protect wounds against water, germs, and dirt, providing clinicians with a more effective tool to fight against surgical-site infections and chronic wounds. Importantly, because the polymer is not a drug, there are no known organisms with tolerance or resistance to Preventogen.
CLINICAL PROOF OF SUCCESS
Preventogen's safety and efficacy were demonstrated in a randomized, prospective trial in deformity correction with external fixators, one of the most infection-prone procedures in medicine, with the Preventogen patients showing 0 infections and the control arm with a 23% infection rate.
"With the use of Preventogen, we are seeing a dramatic decrease in bacterial colonization. We use this product as part of our infection prevention protocol in lower-extremity salvage and reconstruction in high-energy trauma and are also tracking its efficacy vs. superbugs. So far, we are very impressed," commented Dr. Edgardo Rodriguez-Collazo, Director of the Postdoctoral Fellowship Complex Deformity Correction and Microsurgical Limb Reconstruction at Ascension Health.
"Preventogen lays down an impermeable barrier to bacteria, preventing biofilm formation. In our early clinical cases, we have found that chronic wounds thought not to be healable now go on to heal. We see demand for this product growing exponentially as the number of patients with chronic wounds grows annually; clinicians and payors are always looking for ways to heal more wounds in less time," said Dr. Mary E. Hanley DO, FUHM, CWSP, FAPWCA, Medical Director Roper St. Francis Healthcare Wound Care, Hyperbaric Medicine and Limb Preservation Program.
About Preventogen™
Preventogen is a non-antibiotic, FDA-cleared microbicidal liquid agent that actively kills viruses, fungi, and bacteria on contact by lysing the cell membrane, leading to quick cell death. The liquid quickly dries, creating a transparent, biodegradable, odorless, and elastomeric film barrier. The flexible film barrier protects the wound from water, dirt, and oxygen. Carbon dioxide infused into Preventogen during manufacturing is released and lowers the wound bed's pH, which helps promote healing.
We are exploring additional strategic partnerships and market expansion based on robust scientific and clinical data.
About Prevent-Plus
Prevent-Plus, LLC provides innovative infection prevention polymer science used by professional healthcare providers and patients for wound care, infection prevention, and optimized healing across a wide range of clinical applications and specialties.
Contact Information
Brad Chartrand
CEO, Prevent-Plus
[email protected]
(518) 796-8786
Lena Geandreau
Creative Director
[email protected]
(917) 848-0138
Related Images
|
SOURCE: Prevent-Plus, LLC