Novel Combination of Tolerance-Inducing Agent and Human Stem Cell-Derived Islets Demonstrates Functional Insulin Release and Disease Reversal in a Model of Diabetes
MIAMI, FL / ACCESSWIRE / June 24, 2024 / iTolerance Inc. announced today that the Diabetes Research Institute (DRI) at the University of Miami Miller School of Medicine was thrilled to unveil groundbreaking results presented at the American Diabetes Association's (ADA) 84th Scientific Sessions. This innovative approach highlights the potential of human stem cell-derived islets combined with an immunomodulatory microgel to reverse Type 1 Diabetes (T1D). This technology was developed to enable pancreatic islet cells replacement in the allogeneic setting (from a donor to an unrelated recipient) without the need for chronic systemic immunosuppression.
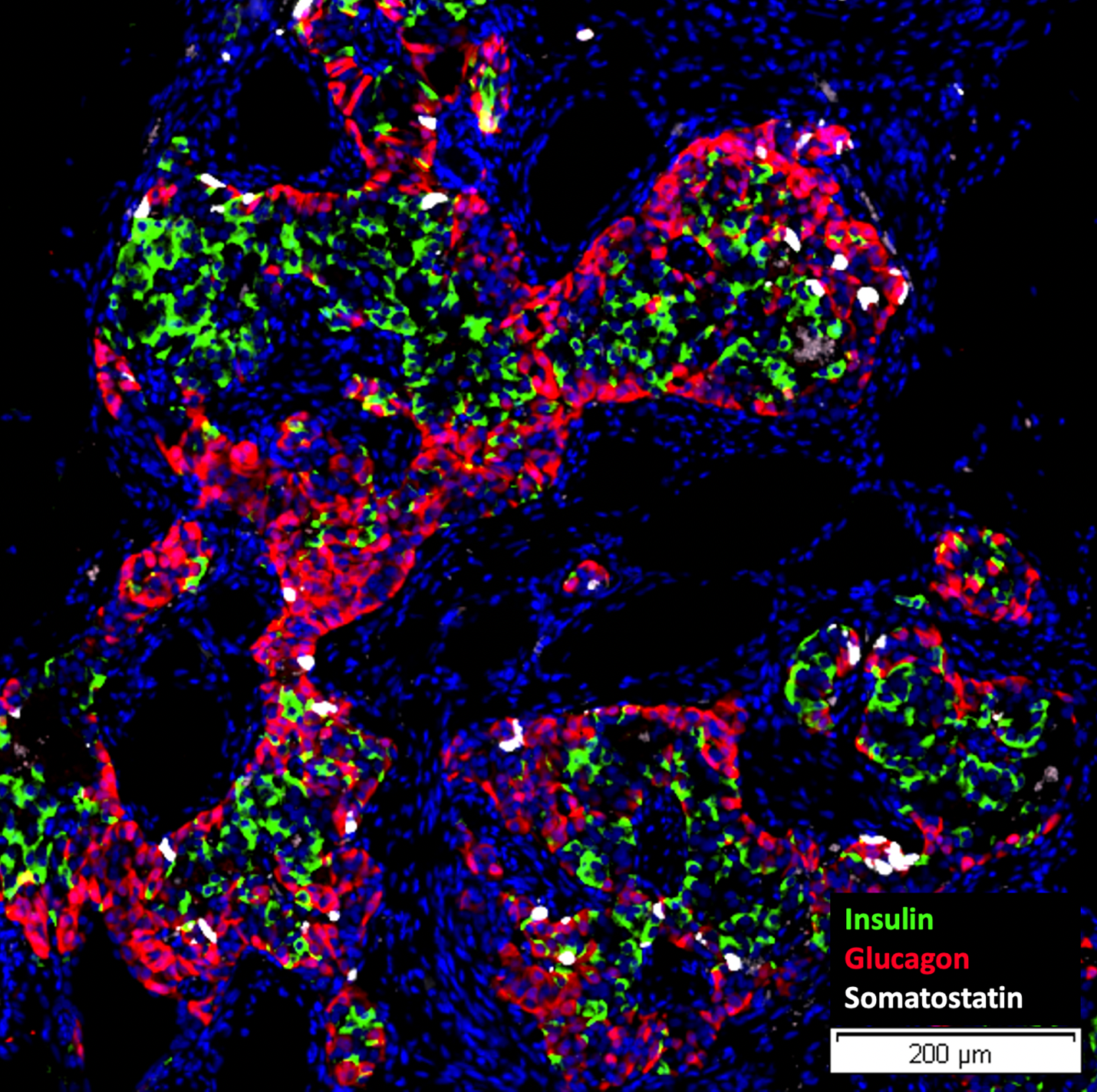
The collaborative effort, spearheaded by Dr. Giacomo Lanzoni`s team at the DRI, together with teams from iTolerance, Inc. and Kadimastem, Ltd, demonstrates that the combination of iTOL-100 engineered microgel developed by iTolerance, Inc. and IsletRx stem cell-derived islets developed by Kadimastem, Ltd. can effectively restore normoglycemia in a model of diabetes.
Dr. Giacomo Lanzoni, PhD, program leader at the DRI, stated, "Our observations highlight the transformative potential of combining stem cell-derived islets with an immunomodulatory microgel. This approach could enable transplantation across the allogeneic barrier, offering a scalable and sustainable solution for T1D, and could enhance the safety and long-term efficacy of islet cell transplantation.
Dr. Camillo Ricordi, MD, Director of the Cell Transplant Center and Director Emeritus at the Diabetes Research Institute, commented: "The Fast Track Center for Testing at the DRI Cell Transplant Center continues to serve as a key shared resource to validate emerging technologies towards a cure for diabetes. We hope to continue to be of assistance towards the identification of reliable and potentially unlimited stem cell-derived islet sources for transplantation, which may one day be able to replace the limited availability of pancreas-derived islets from multiorgan donors, when life-long recipient immunosuppression will no longer be required."
The study's key findings indicate that this combination therapy reverses diabetes and preserves the functional integrity of the transplanted stem cell-derived islets.
Key Highlights:
- iTOL-100, an immunomodulatory microgel developed by iTolerance, Inc., designed to eliminate the need for chronic systemic immunosuppression and shown to induce local immune acceptance of transplanted islets, was found to be compatible with stem cell-derived islets.
- IsletRx, a preparation of Human Stem Cell-Derived Isletsdeveloped by Kadimastem, Ltd., is a scalable and virtually unlimited source of insulin-producing cells and could address the critical shortage of donor islets for transplantation.
- Implantation in a Retrievable Site: The transplantation procedure is performed in a retrievable site, ensuring the possibility of graft retrieval through a minimally invasive surgery, if needed.
- Successful Reversal of Diabetes: The study reports reversal of disease in a chemically induced model of diabetes, with comparable efficacy of IsletRx in the presence or absence of iTOL-100, indicating a lack of toxicity from the microgel.
Additional Comments:
Dr. Anthony Japour, CEO of iTolerance, Inc., commented, "iTolerance is pleased to co-sponsor the project at the Diabetes Research Institute toward a functional cure of T1D through the combination of human stem cell-derived insulin producing islet cells together with our iTOL-100 proprietary immunomodulator. Removing the need for life-long toxic immunosuppressive agents in islet transplantation is a common goal among those working toward a cure for T1D through transplantation without immunosuppression."
Prof. Michel Revel, Chief Scientist of Kadimastem, Ltd., stated: "Our collaboration with iTolerance opens an innovative and world-first avenue for transplanting pancreatic islet cells into people with diabetes without the need for full suppression of the immune system, which is required today in organ transplants." Prof. Revel emphasized, "Our company produces high-quality pancreatic islet cells. The joint data collected by us prove the possibility of combining our cells with the material that locally prevents the rejection of the implant developed by our project partner iTolerance. Having successfully completed an Interact meeting with the FDA, the two companies are moving together to the pre-IND submission stage."
IsletRx is comprised of clinical-grade clusters of human pancreatic islet like cells (ILCs) with the ability to secrete insulin. IsletRx cells can detect the sugar levels in the body and produce the required amounts of insulin and glucagon. The company's technology is unique with its ability to select and enrich only the highest functioning and purest islet cells from the population of pluripotent stem cells which enables the maximum therapeutic effect.
The project was supported in part by iTolerance, Inc., Kadimastem, Ltd., grants funded by the Breakthrough T1D Foundation (formerly known as JDRF) and the Israel-U.S. Binational Industrial Research (BIRD) Foundation.
About the Diabetes Research Institute (DRI): The Diabetes Research Institute at the University of Miami Miller School of Medicine is a recognized world leader in cure-focused research. The DRI is committed to developing and rapidly applying innovative strategies to restore natural insulin production and normalize blood sugar levels without imposing other risks.
For more information, please visit https://diabetesresearch.org/
This breakthrough project will be a cornerstone in our quest to erase Type 1 Diabetes and improve the lives of millions worldwide. The Diabetes Research Institute remains dedicated to advancing the science of diabetes cure and translating these discoveries into practical applications.
About About iTolerance, Inc.
iTolerance is an early-stage privately-held regenerative medicine company developing technologies to enable tissue, organoid or cell therapy without requiring life-long immunosuppression. Leveraging its proprietary biotechnology-derived Streptavidin-FasL fusion protein/biotin-PEG microgel (SA-FasL microgel) platform technology, iTOL-100, iTolerance is advancing a pipeline of programs using both allogenic pancreatic islets and stem cells that have the potential to cure diseases. Utilizing iTOL-100 to induce local immune tolerance, the Company is developing its lead indication as a potential cure for Type 1 Diabetes without the need for life-long immunosuppression. Additionally, the Company is developing iTOL-201 for treating liver failure by utilizing hepatocytes and iTOL-401 as a nanoparticle formulation for large organ transplants without the need for life-long immunosuppression. For more information, please visit itolerance.com.
References:
Rech Tondin A, Yolcu E.S., Herring S.K., Hester D.M., Gadea Y.; Vega G, Szust J, Revel A, Shirwan H, Garcia A, Japour A, Revel M, Molakandov K, Ricordi C, Lanzoni G. Reversal of diabetes by human stem cell-derived islets implanted with an immunomodulatory microgel in a retrievable site. 2024-LB. American Diabetes Association's (ADA) 84th Scientific Sessions, June 21, 2024 - Orlando, FL, USA.
Rech Tondin A, Yolcu E.S., Herring S.K., Hester D.M., Gadea Y.; Vega G, Szust J, Revel A, Shirwan H, Garcia A, Japour A, Revel M, Molakandov K, Ricordi C, Lanzoni G. 2024-LB: Reversal of Diabetes by Human Stem Cell-Derived Islets Implanted with an Immunomodulatory Microgel in a Retrievable Site. Diabetes 2024;73(Supplement_1):2024-LB https://doi.org/10.2337/db24-2024-LB
https://diabetesjournals.org/diabetes/article/73/Supplement_1/2024-LB/156035
Investor Contact
Jenene Thomas
Chief Executive Officer
JTC Team, LLC
T: 833.475.8247
[email protected]
Media Contact
Susan Roberts
T: 202.779.0929
[email protected]



